Dermagraft for Diabetic Foot Ulcers
Dermagraft FAQ
What is Dermagraft used for?
Dermagraft has been used more to stimulate wound healing in chronic wounds such as diabetic foot ulcers than resurface full-thickness acute burn wounds, but has been as successful as split-thickness grafts in the treatment of burns wounds excised to varying depths. 26 Figure 15.8. Commercially available product Dermagraft. From Advanced Biohealing.
How is Dermagraft generated?
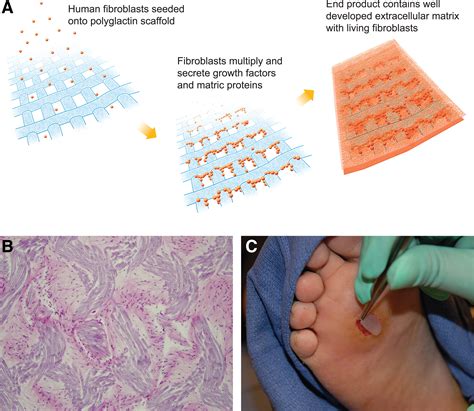
(A) Dermagraft is generated by seeding human dermal fibroblasts onto a polyglactin mesh scaffold. The cells are allowed to expand during which time they lay down a three-dimensional matrix containing collagens type I, III, and VII, various growth factors and cytokines, and numerous proteoglycans.
How does a Dermagraft work?
Dermagraft delivers a collagen-rich living human dermal matrix to the prepared ulcer wound bed. 2 Metabolically active fibroblasts are distributed throughout the Dermagraft and retain the capacity for secreting a variety of regulatory and structural proteins.
What is Dermagraft ® skin substitute?
Dermagraft® is a cryopreserved dermal substitute composed of human fibroblasts, extracellular matrix, and a bioabsorbable scaffold (Figure III.2.6.6 ). FIGURE III.2.6.6. Dermagraft® Skin Substitute (Advanced BioHealing, Westport, CT, USA).
Is Dermagraft a Class 3 medical device?
Dermagraft has been approved by the U.S. FDA as a Class III medical device for use in the treatment of full-thickness DFUs of >6 weeks duration, which extend through the dermis, but without tendon, muscle, joint capsule, or bone exposure.
How effective is Dermagraft?
The effectiveness data are therefore based on the 245 patients with ulcers of greater than 6 weeks duration. The safety analyses were performed on all 314 patients who were randomized into the study. For the population of patients with ulcers of any duration, 17% (27/163) of DERMAGRAFT patients left the study prior to completing it.
Is Dermagraft available in the United States?
DERMAGRAFT has only been available in the United States as an investigational device under IDE G900155. DERMAGRAFT for the treatment of diabetic foot ulcers was approved for sale in Canada in August 1997. DERMAGRAFT was introduced in the United Kingdom in October 1997, and several other European countries, New Zealand and Australia.
Dermagraft References
If you want to know more about Dermagraft, consider exploring links below:
What Is Dermagraft
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3623576/
- https://www.sciencedirect.com/topics/engineering/dermagraft
- https://woundreference.com/app/topic?id=dermagraft
- https://organogenesis.com/pdf/advanced-wound-care-dermagraft-patient-brochure.pdf
- https://www.liebertpub.com/doi/10.1089/wound.2011.0282
- https://diabetesjournals.org/care/article/26/6/1701/26323/The-Efficacy-and-Safety-of-Dermagraft-in-Improving
Dermagraft Information
Explore Related Topics
Nanotherapeutics for Diabetic Wound Healing: A Promising Solution?
How can nanotherapeutics accelerate the healing of diabetic wounds?